Laboratori Ciutadà de Salut Urbana
Planificació urbana, medi ambient i salut

- Durada
- 01/01/2022 - 31/12/2022
- Coordinador
- Celia Santos Tapia
- Finançadors
- Ajuntament de Barcelona - Fundació BIT Habitat
El Laboratori Ciutadà de Salut Urbana és un espai de trobada obert i inclusiu amb el qual volem impulsar el desenvolupament d'idees comuns sota la lògica de l'experimentació i l'aprenentatge col·lectiu. Per mitjà de la participació activa de diversos actors i col·lectius, s'instauraran mecanismes que promoguin la innovació social sota la cultura de proximitat.
Aquest espai neix amb la voluntat de crear comunitats d'aprenentatge i de pràctica, en les quals es treballi en equip per identificar necessitats, problemes i interessos, i impulsar idees creatives que ajudin a millorar les condicions dels barris per mitjà de la cultura científica, el coneixement compartit i l'experimentació amb eines de sensibilització i divulgació. El laboratori propicia condicions favorables a l'escolta, l'observació i la intel·ligència col·lectiva entre participants de diversos perfils vinculats a un mateix context, generant espais de confiança i de cooperació, amb un enfocament col·laboratiu i interdisciplinari.
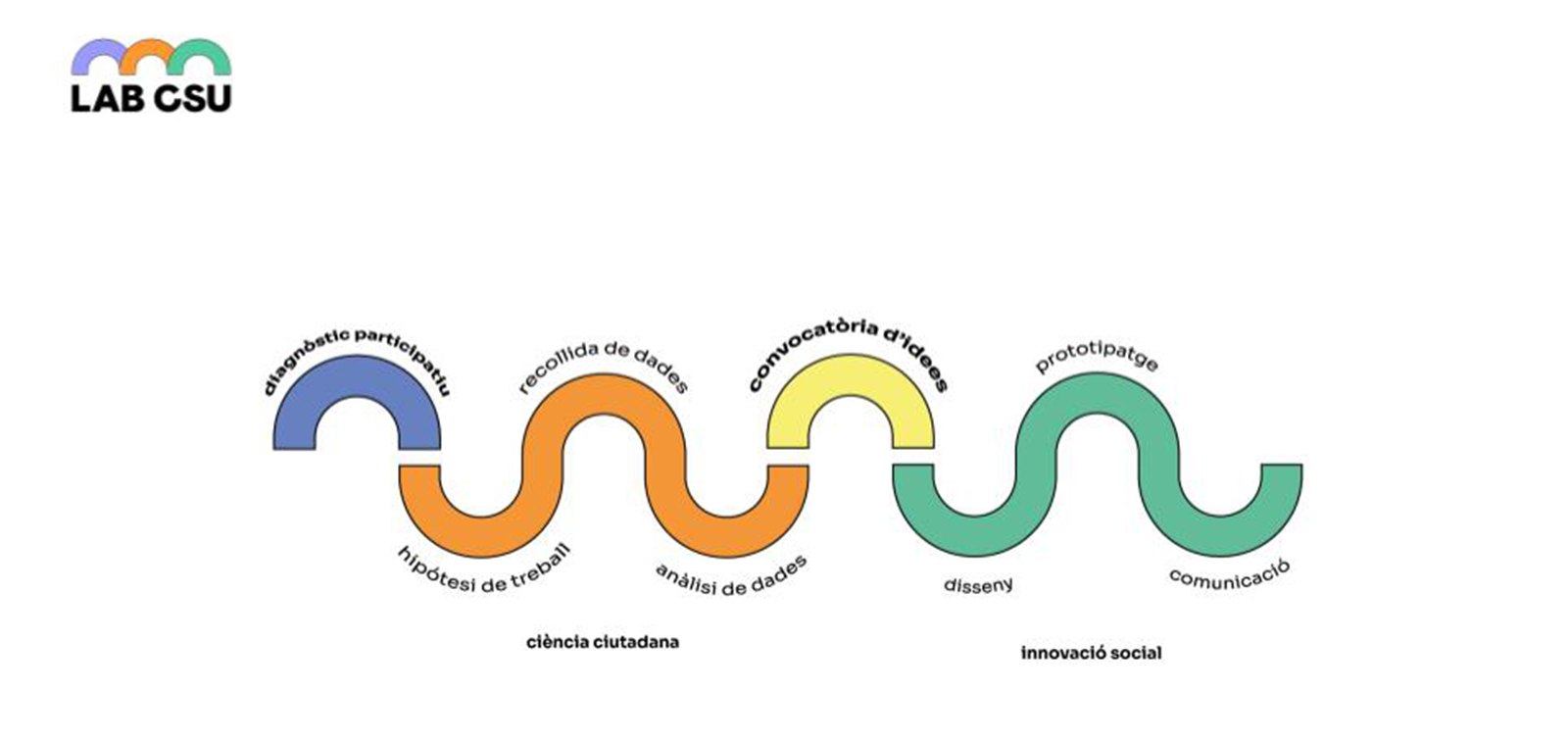
Com funciona?
- Diagnòstic participatiu (etapa 1). Consisteix a identificar i comprendre els principals problemes i necessitats del territori, per mitjà de processos de cartografies col·laboratives. [Consulta'l, properament]
- Procés de ciència ciutadana amb sensors mediambientals oberts (etapa 2). [Consulta'l, properament]
- Convocatòria oberta (etapa 3). En aquest punt es recullen propostes i solucions proposades per la ciutadania, i se'n triaran les propostes guanyadores.
- Disseny i prototipatge (etapa 4). Les propostes i solucions triades es treballen de manera col·laborativa des de les lògiques de l'experimentació i el prototipatge. En aquest punt, entren en joc els laboratoris de fabricació digital o la Xarxa d’Ateneus de Fabricació. Es dóna prioritat al procés per davant del resultat, i es brinda autonomia als grups per promoure la continuïtat dels projectes. [Consulta la convocatòria de col·laboradors]
- Comunicació (etapa 5). Finalment, es treballa en la posada en comú, la presentació i l’explotació dels resultats de cara a la divulgació dels reptes presentats.
De manera transversal, al llarg de tot el procés, es té en compte la documentació de fets i aprenentatges per a la generació col·lectiva de coneixement. És a dir que posteriorment es pugui fer circular —sota el principi de la cultura lliure per a poder ser copiada, distribuïda, modificada i millorada— i resulti útil en altres contextos. S'abordaran diferents disciplines, com la ciència, la tecnologia, la innovació, l'urbanisme i l'art, i s'establiran interrelacions per generar solucions interdisciplinàries.
Finançament total
13.595 €
A través de l'Ajuntament de Barcelona i la Fundación BIT Habitat.
Coorganitzat per:
Projecte CIRCULATE
Our Team
Coordinadora a ISGlobal
-
Celia Santos Staff Scientist
Equip ISGlobal
-
Marina Tarrús Tècnica de divulgació científica de la iniciativa RAM
-
Raül Torán Responsable de Divulgació Científica
-
Mònica Ubalde Investigadora postdoctoral
Altres projectes
Veure projectes passatsMCC-Spain
Estudi multi-cas control poblacional, incloent tumors d'alta incidència a Espanya
Projecte INMA - Infància i Medi Ambient
HELIX
Novel tools for integrating early-life environmental exposures and child health across Europe
EGG/EAGLE
Early Genetics Growth/Early Genetics and Lifecourse Epidemiology
PACE
Pregnancy and Childhood Epigenetics
LIFECYCLE
Early-life stressors and LifeCycle health
OMEGA-NET
Network on the Coordination and Harmonisation of European Occupational Cohorts
BiSC (Barcelona Life Study Cohort)
HARMONIC
Health effects of cArdiac fluoRoscopy and mOdern radIotherapy in paediatriCs
Mobilise-D
Connecting digital mobility assessment to clinical outcomes for regulatory and clinical endorsement
EARLY-ADAPT
Signs of Early Adaptation to Climate Change
COVICAT
Cohorte Covid-19 en Cataluña
ATHLETE
Advancing Tools for Human Early Lifecourse Exposome Research and Translation
CONTENT
Cohort de COVID-19 a Espanya: dinàmica social, salut mental i desigualtats
EUCAN-Connect
A federated FAIR platform enabling large-scale analysis of high-value cohort data connecting Europe and Canada in personalized health
OBERON
An integrative strategy of testing systems for identification of EDs related to metabolic disorders
EXPANSE
EXposome Powered tools for healthy living in urbAN SEttings
AURORA 2021
Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics
ONES
Fine Particle Matter, Fetal Growth, and Neurodevelopment: Examining Critical Windows of Susceptibility
AIR-NB
Pre-natal exposure to urban AIR pollution and pre- and post-Natal Brain development
El impacto de la exposición al metaboloma de esteroides materno-fetales en el crecimiento infantil y los resultados neurológicos (IGRO)
Project Code: PI21/01269
NutinBrain
The role of seafood and nut consumption on human neurodevelopment from pregnancy to adolescence
ALTER - Contaminación del aire, microbiota intestinal y neurodesarrollo en los primeros 24 meses de vida
Project Code: PI21/01278
Alimentación S2: por una dieta saludable y sostenible
Estudi sobre l'exposició a nano i microplàstics a través de l'aigua de consum de Barcelona
És millor consumir l'aigua d'aixeta si volem reduir l'exposició a nano/microplàtics?
UrbanKids
Urban and social environment and childhood obesity – a natural moving2health experiment
Characterizing Oral Exposure to Nanoplastics and Microplastics
Characterization of Oral NMP Exposure
iGenCO
In-Depth Genomics and Cross-Omics Analysis for Undiagnosed Rare Diseases on a User-Friendly Collaborative Platform
5G expOsure, causaL effects, and rIsk perception through citizen engAgemenT
GOLIAT
CityExposomeCat
An Exposome Approach to Urban Health: Individualized Environmental Exposure Assessment in an Adults Population Cohort Study (GCAT)
TwinAir
Digital Twins Enabled Indoor Air Quality Management for Healthy Living
Subclinical Infections in Children and Long Term Health Effects
Infection acquisition in early life and health outcomes in childhood - MARATO TV3
Exposición prenatal a sustancias poli y perfluoradas en agua de consumo y neurodesarrollo en el inicio de la vida
Project Code: PI20/00829
Base genética materna y fetal de la función placentaria
Project Code: PI20/01116
TOLIFE
Combining Artificial Intelligence and smart sensing TOward better management and improved quality of LIFE in chronic obstructive pulmonary disease
BWater
Drinking Water in Barcelona: Sustainability and Health Impact Assessment
CUPID
intoDBP
Innovative Tools to Control Organic Matter and Disinfection Byproducts in Drinking Water
FINDOOR
FTIR spectroscopy for real-time detection of bacterial outbreaks and the rapid identification of pathogenic serotypes, relapsing infections and antibiotic resistance
OccRF-Health
Occupational exposure to radiofrequency electromagnetic fields: From exposure assessment to study of health in workers and their offspring
EPHOR
Exposome Project for Health and Occupational Research
EXPONIT
Analysing and studying how night shift work affects workers' circadian rhythms and health
IHEN
International Human Exposome Network
El microbioma intestinal y la disrupción circadiana
Un estudio epidemiológico molecular sobre enfermedades cardiometabólicas y salud mental
B-Triage
Una prueba en el punto de atención para la estratificación del riesgo de los pacientes febriles basada en los niveles de sTREM-1
e-QuoL
e-health tools to promote Equality in Quality of Life for childhood to young adulthood cancer patients, survivors and their families
AM-MENTAL
Com afecta la teva salut mental que el teu cap sigui un algoritme?
UBDPOLICY
The Urban Burden of Disease Estimation for POLICY Making





