SMV Delivery
Determining the optimal approach to deliver a malaria vaccine in seasonal transmission areas through phase 4 implementation research
- Durada
- 01/10/2024 - 01/04/2028
- Coordinador
- Cristina Enguita-Fernàndez
- Finançadors
- EDCTP3
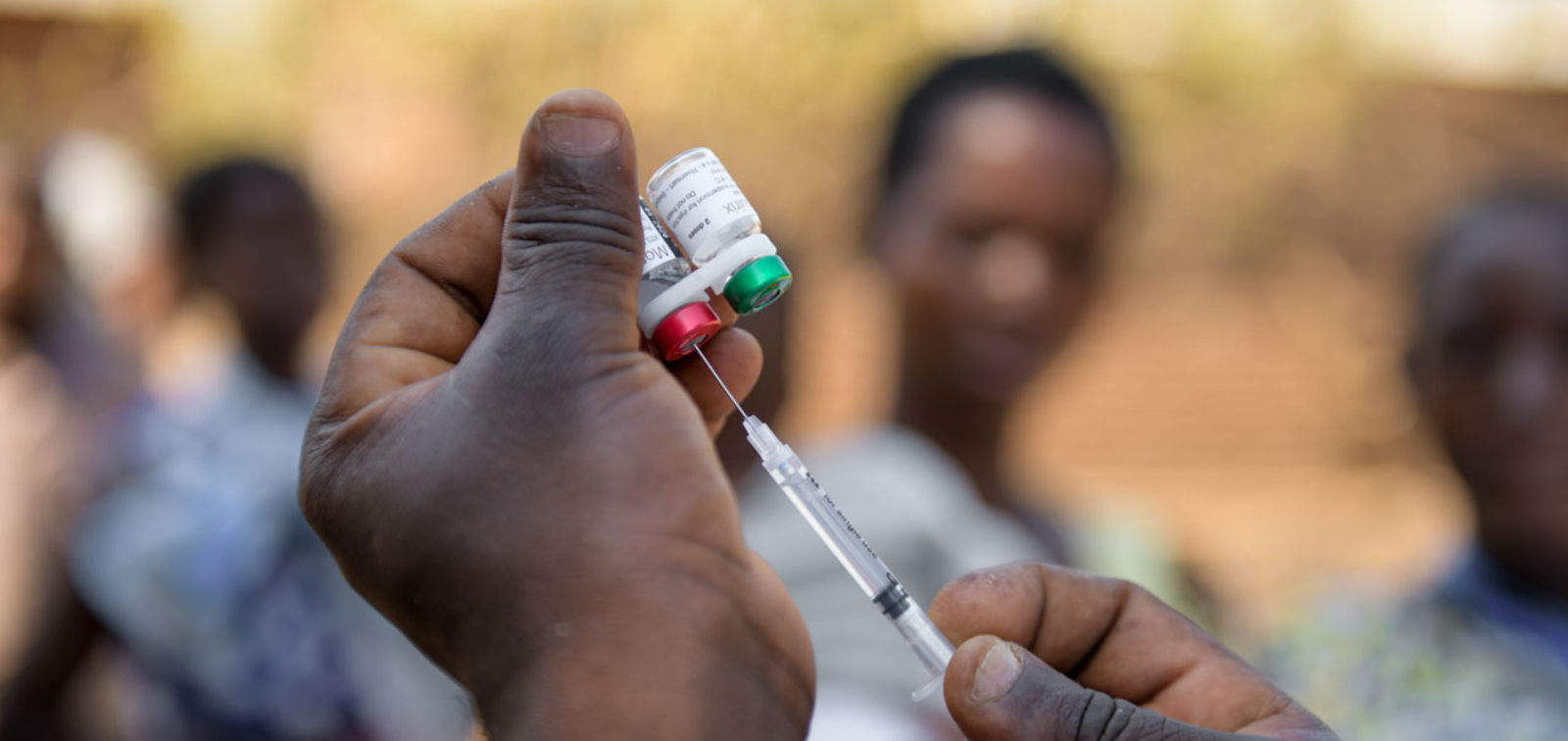
Seasonal malaria chemoprevention (SMC) — the administration of sulphadoxine-pyrimethamine and amodiaquine (SP +AQ) — during the peak period of malaria risk has proved to be a highly effective malaria control measure in areas where transmission of malaria is highly seasonal. SMC was administered to 45 million children in 2021. Still, malaria remains the main cause of hospital admissions among young children in many parts of the Sahel and sub-Sahel and additional control measures are needed to protect children.
The malaria vaccines RTS,S/AS01E, and R21/Matrix provide a high level of protection during the first few months after a primary series of vaccination, or after a booster dose, but efficacy wanes progressively during the following months and years. Thus, in areas with seasonal malaria transmission, one potential use for these vaccines, which provide a high but relatively short period of protection, is administration of an annual booster dose given prior to the malaria transmission season in children who have received three priming doses of the vaccine in the first year of life. However, there is a debate about the best approach to delivery of the booster doses.
The main objective of this study is to determine whether annual booster doses of the malaria vaccines administered pre-transmission season through a mass campaign will achieve better coverage and higher impact on the incidence of malaria than pre-transmission booster doses delivered by an EPI programme at vaccination centres.
This will be a pragmatic implementation study involving two cohorts of children. Two districts that have comparable malaria epidemiology, social structure, coverage of EPI vaccines, and access to treatment will be identified in both Mali and in Guinea from the list of districts that are selected for roll out of R21M vaccine by the Ministry of Health. The study will compare different delivery strategies — season-based vs. age-based approaches and mass community campaigns vs. health facility-based distribution — to determine the most effective method. Within this project, the MCRH program at ISGlobal is leading the social and implementation sciences work package.
Total Funding
4.352.355€
Our Team
Coordinación
-
 Cristina Enguita Assistant Research Professor
Cristina Enguita Assistant Research Professor
Equipo ISGlobal
-
 Raquel González Associated Researcher
Raquel González Associated Researcher -
 Clara Menéndez Research Professor, Directora de la Iniciativa i del Programa de Salut Materna, Infantil i Reproductiva
Clara Menéndez Research Professor, Directora de la Iniciativa i del Programa de Salut Materna, Infantil i Reproductiva -
Maria Martínez Assistent d'investigació
Altres projectes
Veure projectes passatsCaDMIA-plus
Validación continuada de la autopsia mínimamente invasiva (MIA) para la investigación de la causa de muerte en niños pequeños, y desarrollo de un centro de investigación y formación sobre el estudio de la causa de muerte
MAMAH
Improving Maternal and Infant Health by reducing malaria risks in African women: evaluation of the safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in HIV-infected pregnant women
MIBio
Identification of Prematurity and Pre-Eclampsia as Causes of Mortality
MA-CoV
Prevalence and impact of SARS-CoV-2 infection on maternal and infant health in African populations
IPERVAC-SL
Impact of perceptions of COVID-19 vaccines on health-seeking behaviours in Sierra Leone
RESPIRE
Respiratory syncytial virus (RSV) in African pregnant women and children
VITAL
Towards preparedness for new maternal vaccinations: Understanding barriers and facilitators to maternal vaccine acceptance
SARA (SARS-CoV-2 and Acetylsalicylic acid)
Efficacy of low dose acetylsalicylic acid in preventing adverse maternal and perinatal outcomes in SARS-CoV-2 infected pregnant women
HIV drug resistance (HIVDR)
Description of HIV drug resistance patterns and its association with the risk of HIV mother to child transmission among pregnant women from southern Mozambique
Evaluación de la eficacia del ácido acetilsalicílico a dosis bajas en la prevención de los efectos maternos y perinatales adversos en mujeres embarazadas infectadas por el SARS-CoV-2
PROTECT
PReparing for Optimal Phase III/IV maTErnal Group B StreptococCal vaccine Trials in Africa
ICARIA
Improving Care through Azithromycin Research for Infants in Africa
MULTIPLY
MULTIple doses of IPTi Proposal: a Lifesaving high Yield intervention




